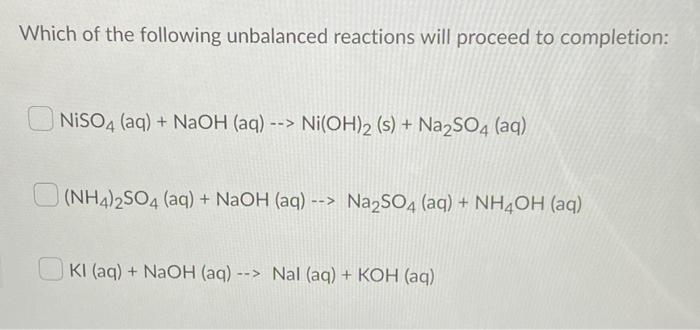
Figure 2 from Polyol-mediated synthesis of mesoporous α-Ni(OH)2 with enhanced supercapacitance. | Semantic Scholar

Direct preparation of Al-substituted α-Ni(OH)2 from Al-containing salt solution by immersing method - ScienceDirect
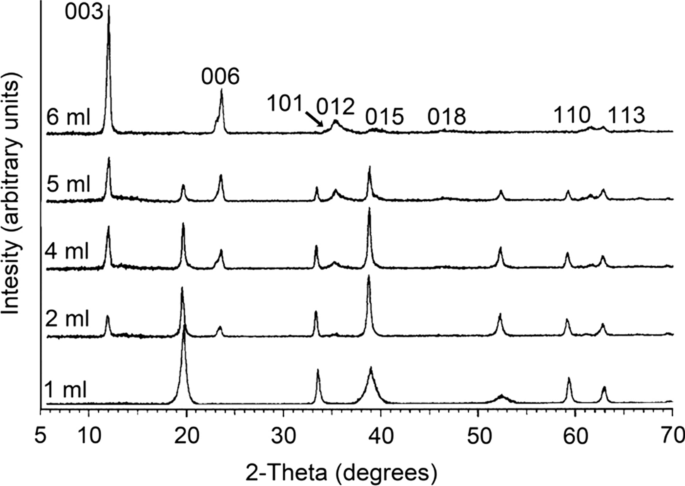
![PDF] Effect of reaction conditions on size and morphology of ultrasonically prepared Ni(OH)(2) powders. | Semantic Scholar PDF] Effect of reaction conditions on size and morphology of ultrasonically prepared Ni(OH)(2) powders. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/71d4ed12a62695a24100135a87df3664a7845d6e/2-Figure1-1.png)
PDF] Effect of reaction conditions on size and morphology of ultrasonically prepared Ni(OH)(2) powders. | Semantic Scholar

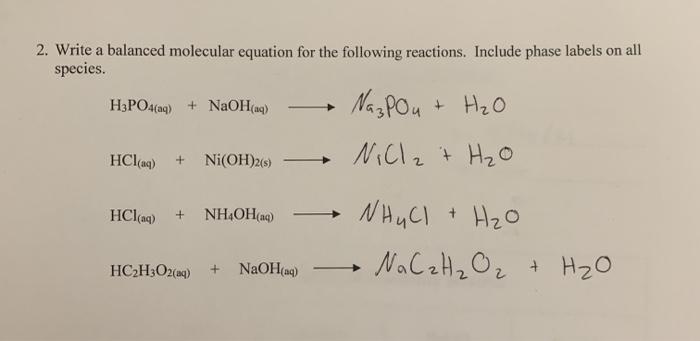
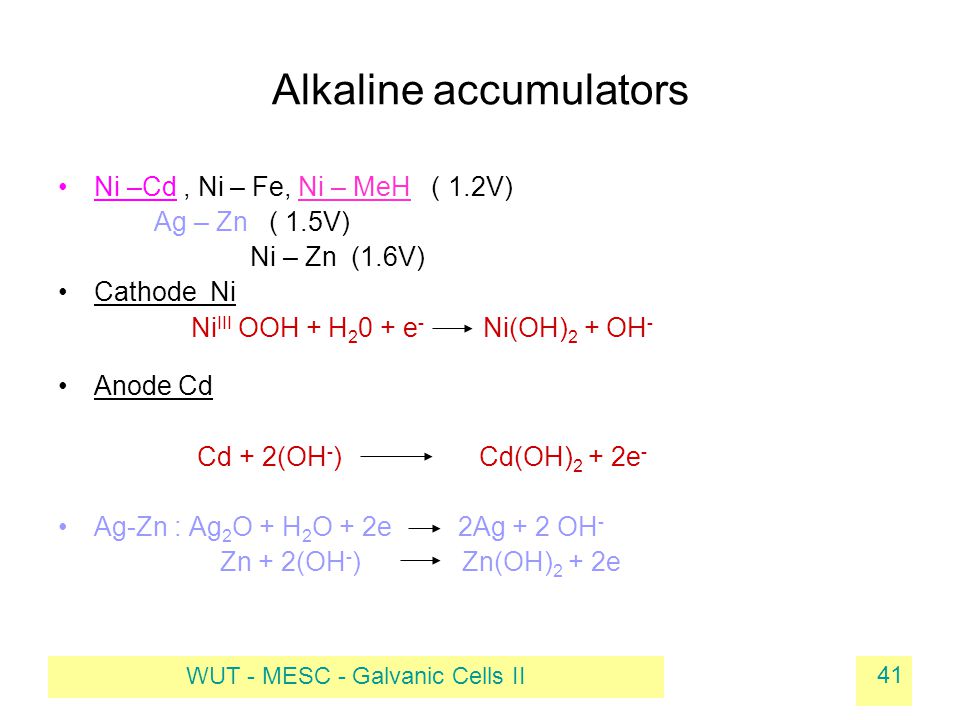
E740: Equilibrium – Complex Ions – Metal + Ammonia Complexes | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

5 Six methods of preparing Ni(OH) 2. (a) Basification of a nickel(II)... | Download Scientific Diagram

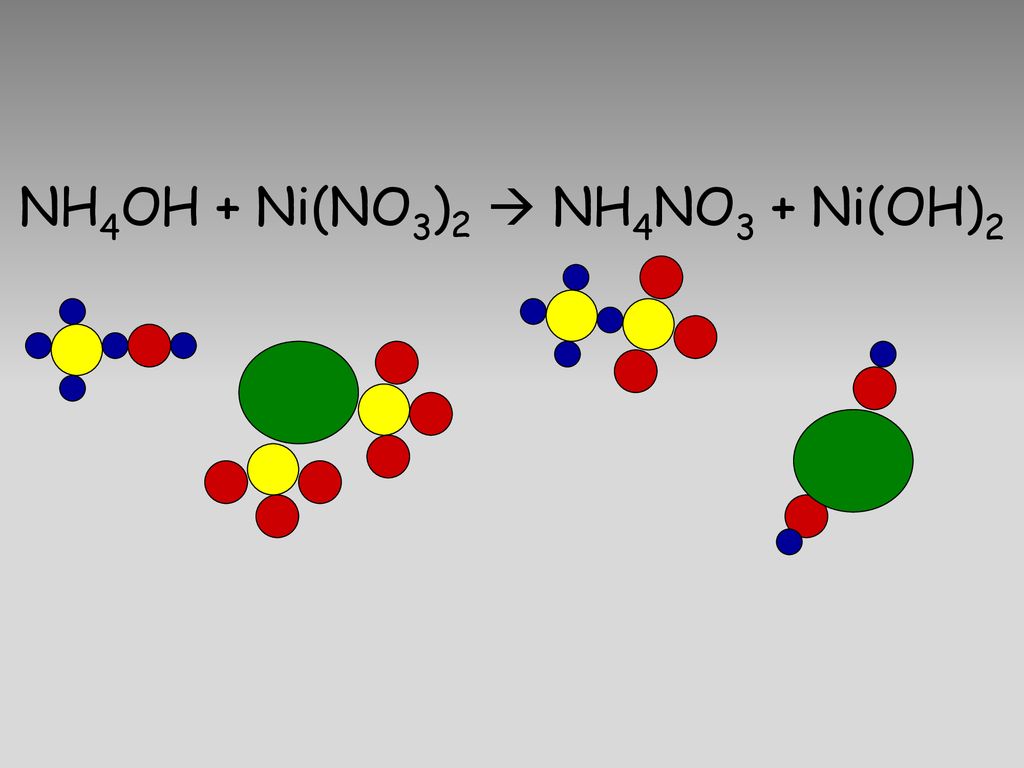
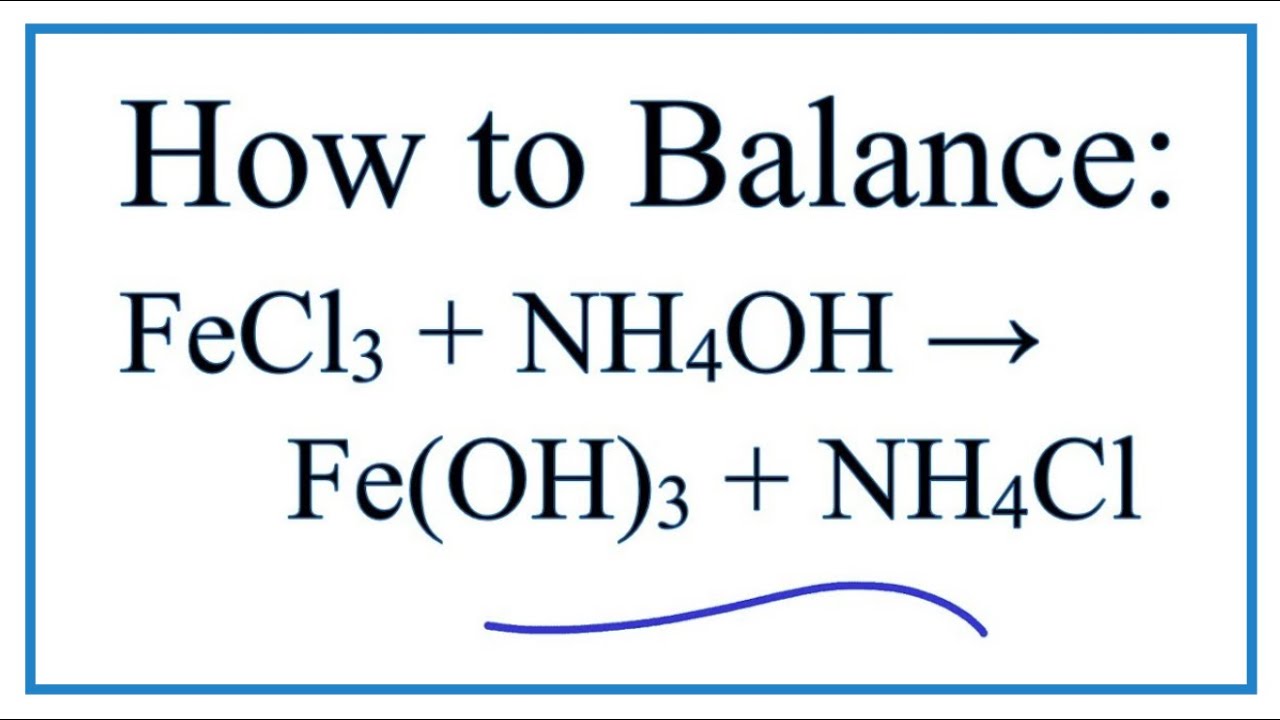
Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect

TGA/DTA thermograms of Ni(OH) 2 prepared (a) without and (b) with CTAB. | Download Scientific Diagram